Chemquest 42 moles and reactions – Prepare to embark on a captivating journey through the realm of chemistry as we delve into ChemQuest 42: Moles and Reactions. This comprehensive exploration unravels the mysteries of chemical transformations, guiding you through the intricacies of stoichiometry, balancing equations, and reaction rates.
Discover the fundamental concept of a mole, the cornerstone of quantitative chemistry. Understand the significance of Avogadro’s number and master the art of converting between moles and mass. Dive into the fascinating world of chemical equations, learning how to balance them effortlessly, ensuring the conservation of mass.
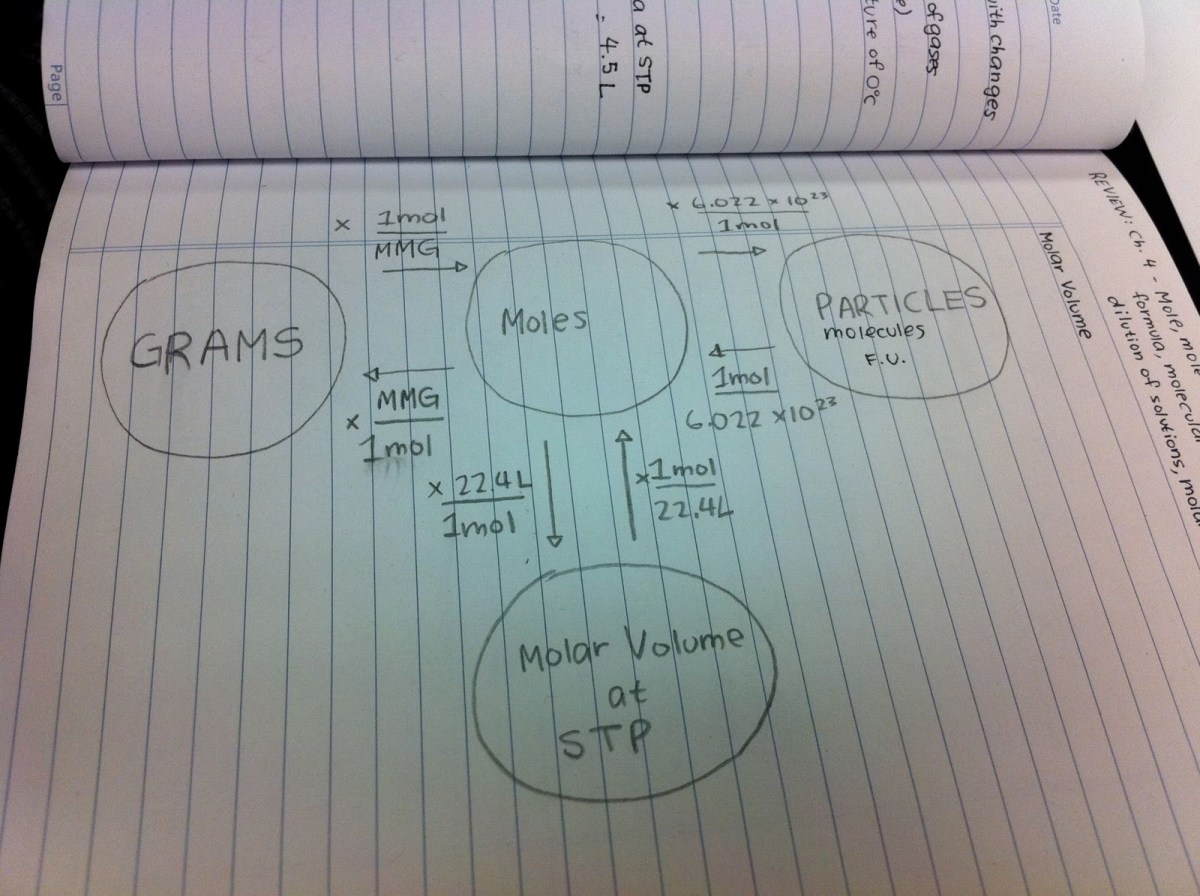
Mole Conversions
A mole is the fundamental unit of measurement for the amount of a substance. It is defined as the amount of a substance that contains exactly 6.02214076×10 23elementary entities. These entities can be atoms, molecules, ions, or electrons.
Avogadro’s number is the numerical value of the amount of entities in one mole of a substance. It is named after the Italian scientist Amedeo Avogadro, who first proposed the concept of a mole in 1809.
Converting Between Moles and Mass
To convert between moles and mass, you can use the following steps:
- Find the molar mass of the substance. The molar mass is the mass of one mole of the substance, expressed in grams per mole (g/mol).
- Multiply the molar mass by the number of moles to find the mass of the substance.
- Alternatively, divide the mass of the substance by the molar mass to find the number of moles.
Mass and Number of Moles for Various Substances
The following table shows the mass and number of moles for various substances:
| Substance | Mass (g) | Number of Moles |
|---|---|---|
| Water (H2O) | 18.015 | 1 |
| Sodium chloride (NaCl) | 58.44 | 1 |
| Glucose (C6H12O6) | 180.16 | 1 |
| Carbon dioxide (CO2) | 44.01 | 1 |
| Nitrogen (N2) | 28.01 | 1 |
Balancing Chemical Equations
A balanced chemical equation shows the exact number of atoms of each element on both sides of the equation, ensuring that the law of conservation of mass is upheld. This law states that matter cannot be created or destroyed in a chemical reaction, only rearranged.
To balance an equation, coefficients are added to the reactants and products to ensure that the number of atoms of each element is equal on both sides.
Method for Balancing Chemical Equations
- Write the unbalanced equation.Identify the reactants and products involved in the reaction.
- Start by balancing the elements that appear in the smallest number of molecules.For example, if there is only one molecule of a reactant or product, balance that element first.
- Balance the elements one at a time.Choose an element that appears in multiple molecules and adjust the coefficients until the number of atoms of that element is equal on both sides.
- Check the balance of all elements.Once one element is balanced, check the balance of the remaining elements and adjust coefficients as needed.
- Balance the charges.If the equation involves ions, ensure that the total charge on the reactants is equal to the total charge on the products.
Stoichiometry
Stoichiometry is the branch of chemistry that deals with the quantitative relationships between reactants and products in chemical reactions. It is essential for predicting the amount of reactants and products involved in a reaction, as well as for calculating the limiting reactant.
The stoichiometric ratio is the ratio of the moles of reactants and products in a chemical reaction. It is determined by the coefficients in the balanced chemical equation. For example, in the reaction 2H 2+ O 2→ 2H 2O, the stoichiometric ratio of hydrogen to oxygen is 2:1, and the stoichiometric ratio of water to hydrogen is 1:2.
Limiting Reactant
The limiting reactant is the reactant that is completely consumed in a chemical reaction. To determine the limiting reactant, compare the moles of each reactant to the stoichiometric ratio. The reactant with the smallest mole ratio is the limiting reactant.
For example, consider the reaction between 2 moles of hydrogen and 1 mole of oxygen. The stoichiometric ratio of hydrogen to oxygen is 2:1. Comparing the moles of each reactant to the stoichiometric ratio, we find that the mole ratio of hydrogen is 2/2 = 1, and the mole ratio of oxygen is 1/1 = 1. Since the mole ratio of hydrogen is smaller, hydrogen is the limiting reactant.
Stoichiometric Ratios
The following table summarizes the stoichiometric ratios for some common chemical reactions:
| Reaction | Stoichiometric Ratio |
|---|---|
| 2H2 + O2 → 2H2O | 2:1 |
| CH4 + 2O2 → CO2 + 2H2O | 1:2 |
| 2Na + Cl2 → 2NaCl | 2:1 |
| Fe + 2HCl → FeCl2 + H2 | 1:2 |
Types of Chemical Reactions
Chemical reactions can be classified into different types based on the changes that occur during the reaction. The main types of chemical reactions are synthesis, decomposition, single displacement, double displacement, and combustion.
Synthesis Reactions
Synthesis reactions involve the combination of two or more substances to form a new, more complex substance. The general form of a synthesis reaction is:“`A + B → AB“`where A and B are the reactants and AB is the product.Example:“`
H₂ + O₂ → 2H₂O
“`In this reaction, hydrogen and oxygen combine to form water.
Decomposition Reactions, Chemquest 42 moles and reactions
Decomposition reactions are the opposite of synthesis reactions. They involve the breakdown of a compound into two or more simpler substances. The general form of a decomposition reaction is:“`AB → A + B“`where AB is the reactant and A and B are the products.Example:“`
H₂O → 2H₂ + O₂
“`In this reaction, water breaks down into hydrogen and oxygen.
Single Displacement Reactions
Single displacement reactions involve the replacement of one element in a compound by another element. The general form of a single displacement reaction is:“`A + BC → AC + B“`where A is the displacing element, B is the element being displaced, and AC is the new compound formed.Example:“`Fe
+ CuSO₄ → FeSO₄ + Cu“`In this reaction, iron displaces copper from copper sulfate to form iron sulfate and copper.
Double Displacement Reactions
Double displacement reactions involve the exchange of ions between two compounds. The general form of a double displacement reaction is:“`AB + CD → AD + CB“`where A and C are the cations (positive ions) and B and D are the anions (negative ions).Example:“`NaCl
+ AgNO₃ → NaNO₃ + AgCl“`In this reaction, sodium ions and chloride ions exchange places with silver ions and nitrate ions to form sodium nitrate and silver chloride.
Combustion Reactions
Combustion reactions are a type of chemical reaction that involves the rapid reaction of a substance with oxygen, usually producing heat and light. The general form of a combustion reaction is:“`Fuel + O₂ → CO₂ + H₂O + Energy“`where Fuel is the substance being burned, CO₂ is carbon dioxide, H₂O is water, and Energy is heat and light.Example:“`CH₄
+ 2O₂ → CO₂ + 2H₂O + Energy“`In this reaction, methane reacts with oxygen to produce carbon dioxide, water, and energy.
Reaction Rates
The rate of a chemical reaction refers to how quickly the reactants are converted into products. Several factors can influence the reaction rate, including temperature, concentration, surface area, and the presence of a catalyst.
Activation Energy
Activation energy is the minimum amount of energy required for a reaction to occur. When reactants collide, they must have sufficient energy to overcome the activation energy barrier to form products. The higher the activation energy, the slower the reaction rate.
Factors Affecting Reaction Rates
The following table summarizes the effects of temperature, concentration, and surface area on reaction rates:
| Factor | Effect on Reaction Rate |
|---|---|
| Temperature | Increases reaction rate as temperature increases. |
| Concentration | Increases reaction rate as reactant concentration increases. |
| Surface Area | Increases reaction rate as surface area of reactants increases. |
Equilibrium: Chemquest 42 Moles And Reactions
Chemical equilibrium occurs when the forward and reverse reactions of a chemical process happen at the same rate, resulting in no net change in the concentrations of the reactants and products. Equilibrium is a dynamic state where the forward and reverse reactions continuously occur, but the concentrations of the substances involved remain constant.
Factors Affecting Equilibrium
Several factors can influence the equilibrium position of a reaction:
- Concentration:Increasing the concentration of reactants shifts the equilibrium towards the products, while increasing the concentration of products shifts the equilibrium towards the reactants.
- Temperature:Raising the temperature usually shifts the equilibrium towards the endothermic reaction (the reaction that absorbs heat). Conversely, lowering the temperature shifts the equilibrium towards the exothermic reaction (the reaction that releases heat).
- Pressure:For reactions involving gases, increasing the pressure shifts the equilibrium towards the side with fewer moles of gas. Decreasing the pressure shifts the equilibrium towards the side with more moles of gas.
| Factor | Effect on Equilibrium Position |
|---|---|
| Concentration of Reactants | Increasing: Shifts towards productsDecreasing: Shifts towards reactants |
| Concentration of Products | Increasing: Shifts towards reactantsDecreasing: Shifts towards products |
| Temperature | Increasing: Shifts towards endothermic reactionDecreasing: Shifts towards exothermic reaction |
| Pressure (for gases) | Increasing: Shifts towards side with fewer moles of gasDecreasing: Shifts towards side with more moles of gas |
Popular Questions
What is the significance of Avogadro’s number?
Avogadro’s number represents the number of atoms, molecules, or ions present in one mole of a substance, providing a fundamental connection between the macroscopic and microscopic scales.
How do I balance chemical equations?
Balancing chemical equations involves adjusting the coefficients in front of each reactant and product to ensure that the number of atoms of each element is equal on both sides, adhering to the law of conservation of mass.
What is the limiting reactant in a chemical reaction?
The limiting reactant is the reactant that is completely consumed in a chemical reaction, determining the maximum amount of product that can be formed.
How does temperature affect reaction rates?
Increasing temperature generally leads to faster reaction rates as it provides more energy to the reactants, allowing them to overcome the activation energy barrier more easily.
What is chemical equilibrium?
Chemical equilibrium occurs when the forward and reverse reactions in a system proceed at the same rate, resulting in no net change in the concentrations of the reactants and products.