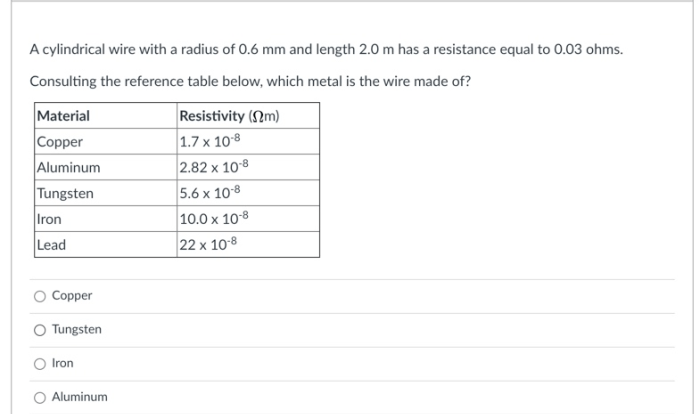
Indium has a tetragonal unit cell, embarking us on a captivating journey into the realm of crystallography. This unique structural arrangement bestows upon indium remarkable properties that set it apart from its metallic counterparts, promising a narrative rich in scientific intrigue and practical applications.
Indium’s tetragonal unit cell, a cornerstone of its atomic architecture, comprises a lattice of atoms arranged in a precise, repeating pattern. This intricate arrangement governs the material’s physical and chemical characteristics, shaping its behavior in fascinating ways.
Introduction to Indium: Indium Has A Tetragonal Unit Cell
Indium is a chemical element with the symbol In and atomic number 49. It is a silvery-white metal that is soft, malleable, and ductile. Indium is found in trace amounts in zinc, lead, and iron ores. It is also found in some minerals, such as indium sulfide (In2S3) and indium phosphide (InP).Indium
is a relatively rare element, with an abundance of about 0.25 parts per million in the Earth’s crust. It is used in a variety of applications, including:
- Soldering and brazing alloys
- Transparent conducting oxides
- Semiconductors
- Nuclear reactors
Physical and Chemical Properties
Indium is a soft, silvery-white metal with a melting point of 156.6 °C and a boiling point of 2072 °C. It is a good conductor of electricity and heat. Indium is resistant to corrosion and tarnishing.Indium is a reactive metal that forms a variety of compounds with other elements.
It is most commonly found in the +3 oxidation state, but it can also form compounds in the +1 and +2 oxidation states. Indium compounds are typically colorless or white.
Crystal Structure of Indium
Indium crystallizes in a tetragonal unit cell, which is a three-dimensional shape that repeats itself throughout the crystal lattice. The tetragonal unit cell has a square base and a height that is different from the length of the sides of the base.
Lattice Parameters and Atomic Arrangement
The lattice parameters of the tetragonal unit cell of indium are a= b= 3.25 Å and c= 4.95 Å. The atomic arrangement within the unit cell is body-centered, meaning that there is one atom at each corner of the unit cell and one atom in the center of the unit cell.
Comparison to Other Crystal Structures
The tetragonal unit cell of indium is similar to the cubic unit cell, but the height of the tetragonal unit cell is different from the length of the sides of the base. The tetragonal unit cell is also similar to the hexagonal unit cell, but the hexagonal unit cell has a hexagonal base instead of a square base.
Properties and Applications of Indium
Indium possesses unique properties that stem from its tetragonal unit cell structure. These properties make indium valuable in various applications.
Electrical Properties
Indium’s tetragonal structure contributes to its exceptional electrical conductivity and low resistivity. This makes it an ideal material for electrical contacts, solder alloys, and semiconductors.
Malleability and Ductility
The tetragonal unit cell allows indium to be highly malleable and ductile. It can be easily shaped and drawn into thin wires or sheets, making it suitable for applications requiring flexibility and formability.
Corrosion Resistance, Indium has a tetragonal unit cell
Indium exhibits excellent corrosion resistance, particularly to acids and alkalis. This property makes it valuable for use in harsh environments, such as chemical processing equipment and marine applications.
Applications
The unique properties of indium have led to its widespread use in various industries, including:
- Electronics:Indium is used in solder alloys, electrical contacts, and semiconductors due to its high electrical conductivity and low resistivity.
- Automotive:Indium is employed in bearings, gaskets, and anti-friction materials due to its malleability and ductility.
- Chemical Processing:Indium’s corrosion resistance makes it suitable for use in equipment exposed to harsh chemicals.
- Medicine:Indium is used in dental alloys and radioisotopes for medical imaging.
Comparison with Other Metals
Indium is one of several metals that crystallize in a tetragonal unit cell. Other metals with this crystal structure include:
- Tin
- Lead
- Zirconium
- Hafnium
- Titanium
These metals share some similarities with indium, such as their relatively low melting points and high electrical conductivity. However, they also have some key differences.
Properties
Indium is a soft, silvery-white metal with a low melting point of 156.6 °C. It is a good conductor of electricity and heat, and it is resistant to corrosion. Tin and lead are also soft, silvery-white metals with low melting points, but they are not as good conductors of electricity or heat as indium.
Zirconium and hafnium are harder, gray metals with higher melting points than indium. They are also good conductors of electricity and heat, but they are not as resistant to corrosion as indium.
Applications
Indium is used in a variety of applications, including:
- Soldering
- Transistors
- Solar cells
- Coatings
Tin and lead are also used in soldering, but they are not as good conductors of electricity as indium. Zirconium and hafnium are used in nuclear reactors and other high-temperature applications.
Advanced Characterization Techniques
Advanced characterization techniques play a pivotal role in unraveling the intricacies of indium’s tetragonal unit cell, providing profound insights into its structural and material properties. These techniques employ sophisticated instrumentation and analytical methods to probe the material at the atomic and molecular level, offering a comprehensive understanding of its composition, morphology, and behavior.
X-ray Diffraction (XRD)
XRD is a non-destructive technique that utilizes X-rays to determine the crystal structure and lattice parameters of materials. By analyzing the diffraction patterns generated when X-rays interact with the sample, XRD can provide precise information about the atomic arrangement, unit cell dimensions, and crystal orientation.
This technique is particularly valuable for characterizing the long-range order and periodicity of indium’s tetragonal unit cell.
Transmission Electron Microscopy (TEM)
TEM is a powerful imaging technique that utilizes a focused electron beam to visualize the microstructure and atomic structure of materials. By transmitting electrons through a thin sample, TEM allows for the observation of defects, grain boundaries, and other structural features at the nanoscale.
This technique is particularly useful for studying the morphology and crystallographic defects within indium’s tetragonal unit cell.
Scanning Probe Microscopy (SPM)
SPM is a family of techniques that use a sharp probe to scan the surface of a material, providing topographic and property information. Atomic force microscopy (AFM) and scanning tunneling microscopy (STM) are two common SPM techniques used to characterize indium’s tetragonal unit cell.
AFM measures the surface topography by detecting the force between the probe and the surface, while STM images the surface by scanning a sharp probe over the surface and measuring the tunneling current.
Advantages and Limitations
Each characterization technique offers unique advantages and limitations:* XRD provides precise information about the crystal structure and lattice parameters but requires crystalline samples.
- TEM offers high-resolution imaging but requires extensive sample preparation and can be destructive.
- SPM techniques provide surface topography and property information but are limited to small sample areas.
By combining these techniques, researchers can obtain a comprehensive understanding of the tetragonal unit cell of indium, enabling the development of advanced materials and applications.
Question & Answer Hub
What is a tetragonal unit cell?
A tetragonal unit cell is a three-dimensional arrangement of atoms characterized by a square base and a rectangular height. It is one of the seven crystal systems in crystallography.
Why is indium’s tetragonal unit cell unique?
Indium’s tetragonal unit cell is unique because it gives indium specific properties, such as its low melting point, high electrical conductivity, and malleability.
What are some applications of indium’s tetragonal unit cell?
Indium’s tetragonal unit cell is used in various applications, including electronics, semiconductors, and alloys.