What aldehyde or ketone might be present? This question lies at the heart of organic chemistry, guiding researchers in identifying and characterizing these crucial functional groups. Aldehydes and ketones, with their carbonyl groups, play pivotal roles in a myriad of chemical reactions, making their detection and analysis essential in various scientific fields.
Delving into the intricacies of aldehydes and ketones, we will explore their distinct chemical structures, unravel the methods employed to detect their presence, and delve into their versatile applications in industry and research. Along the way, we will uncover the nuances that differentiate aldehydes from ketones, enabling us to confidently identify and characterize these ubiquitous compounds.
Identification of Aldehydes and Ketones: What Aldehyde Or Ketone Might Be Present
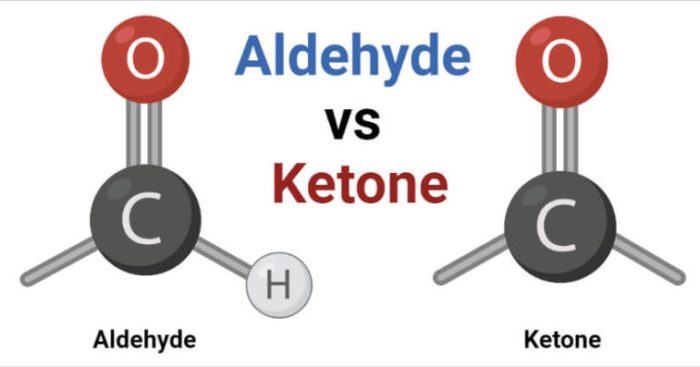
Aldehydes and ketones are organic compounds that contain a carbonyl group (C=O). Aldehydes have the carbonyl group at the end of a carbon chain, while ketones have the carbonyl group in the middle of a carbon chain. This difference in structure gives aldehydes and ketones different chemical properties.Aldehydes
are more reactive than ketones because the carbonyl group in aldehydes is more exposed. This makes aldehydes more likely to undergo nucleophilic addition reactions, which are reactions in which a nucleophile (a species that donates electrons) adds to the carbonyl group.
Ketones, on the other hand, are less reactive than aldehydes because the carbonyl group is less exposed.
Methods for Detecting Aldehydes and Ketones
There are a number of different methods that can be used to detect the presence of aldehydes and ketones. One common method is to use a Tollens’ reagent. Tollens’ reagent is a solution of silver nitrate and ammonia. When Tollens’ reagent is added to an aldehyde, the aldehyde is oxidized to a carboxylic acid and silver metal is deposited.
This reaction can be used to distinguish aldehydes from ketones, as ketones do not react with Tollens’ reagent.Another common method for detecting aldehydes and ketones is to use a Fehling’s reagent. Fehling’s reagent is a solution of copper sulfate and sodium hydroxide.
When Fehling’s reagent is added to an aldehyde or ketone, the aldehyde or ketone is oxidized to a carboxylic acid and copper(I) oxide is deposited. This reaction can be used to distinguish aldehydes from ketones, as aldehydes react with Fehling’s reagent more quickly than ketones.
Chemical Reactions of Aldehydes and Ketones
Aldehydes and ketones undergo a variety of chemical reactions. One common reaction is nucleophilic addition. In a nucleophilic addition reaction, a nucleophile adds to the carbonyl group of an aldehyde or ketone. This reaction can be used to synthesize a variety of different compounds, including alcohols, ethers, and amines.Another
common reaction of aldehydes and ketones is oxidation. In an oxidation reaction, the carbonyl group of an aldehyde or ketone is oxidized to a carboxylic acid. This reaction can be used to synthesize carboxylic acids, which are used in a variety of industrial and commercial applications.
Applications of Aldehydes and Ketones, What aldehyde or ketone might be present
Aldehydes and ketones are used in a variety of industrial and commercial applications. Aldehydes are used in the production of plastics, synthetic fibers, and fragrances. Ketones are used in the production of solvents, pharmaceuticals, and food additives.
User Queries
How can we differentiate between aldehydes and ketones?
Aldehydes possess a hydrogen atom directly bonded to the carbonyl group, while ketones have two alkyl or aryl groups attached to the carbonyl group.
What are the common methods used to detect aldehydes and ketones?
Various methods are employed, including Tollens’ test, Fehling’s test, Benedict’s test, and the 2,4-dinitrophenylhydrazine (2,4-DNP) test.
What are some industrial applications of aldehydes and ketones?
Aldehydes and ketones find widespread use in the production of plastics, fragrances, flavors, and pharmaceuticals.